THE
SCIENTIFIC METHOD
or
The
of the Temperamental Aluminum Can
Source: Nissani, Maier, Shifrin, The Physics Teacher, vol. 32,
pp. 104-107 (1994)
Note to Science Teachers: Copy the Students Manual below for your
students, not the background materials which follow it!
Note: this exercise stresses the commonsense aspect of
science. Scientists sometimes use a special vocabulary which is not readily comprehensible
to novices. As a result, upon first picking up a specialized text in physics we may feel
discouraged. We go through an experience similar to that of immigrants who first land in
America without knowing a single word of English. Despite the hardships, most immigrants
overcome this difficulty because they know that there is nothing mysterious about the
English language. It is inaccessible for a while, but with determination, self-confidence,
and hard work, they too will be able to achieve fluency in that new language. The same
goes for physics. The notion that most people can't grasp what it's all about is entirely
mistaken. You can understand and enjoy physics, if you just give yourself a chance.
Most physics texts and lectures often
describe the way others solved scientific mysteries. In contrast, this exercise will try
to show that you too can in principle solve scientific puzzles on your own, if you just
are curious enough about the world around you. At this point, you are not, probably,
sufficiently familiar with what is already known to make unique contributions to physics.
So, as a second best, this exercise will guide you along a path of rediscovering on your
own a few facts and explanations which are already well accepted in the scientific
community, but which are, most likely, new to you.
Thus, the main point of this take-home exercise is rediscovery
--finding out answers on your own. If you look up answers beforehand,
you will deny yourself the fun, excitement, and sense of empowerment which come from
standing on your own two feet. Also, please submit your report on time, before the subject
is discussed in class!
Needed Materials: A few empty aluminum
soda cans, a big bowl filled with cold water, a nail, a small drinking glass, a heat
source (cooking stove or a hot plate), tongs (or a pot holder), a 3-inch by 5-inch index
card.
A Thought Experiment. This initial
step does not entail any operations, except thinking and writing down your answer. Simply
try to grasp the basic setup and figure out what would happen.
Imagine that you conduct the following experiment. You pour a small amount of water
into an empty aluminum soda can
--one inch or so from the
bottom of the can. You then place the can on a burner or hot plate and bring the water to
a boil. You grab the bottom of the can with a pair of tongs, invert the can (holding it
upside down, with the opening at the bottom), and QUICKLY immerse it in a large bowl of
cold water.
Assignment #1: Please begin your lab
report by writing down now what you think may happen? Why?
Note:
Don't revise your guesses later,
after you know the answer.
Assignment #2: Actually carry out this
experiment and describe what happened.
Note: If you follow the instructions, something dramatic should happen. If it doesn't,
carefully re-read the instructions and repeat the experiment. If nothing works, call your
instructor or one of your fellow students and request their help.
You have just completed two steps in your apprenticeship as a physicist. You have
observed something rather unexpected, and you are curious
--you have to be. You are not content to leave the subject alone, with
question marks dangling all over it. You want to understand why this happens. Can you
think of an explanation for the can's behavior? Please note: The only bad explanation is
no explanation. Anything else is fair game.
Hint: When the water is boiled inside the can, it turns into steam. Steam is a gas
which gradually displaces the air in the can. When steam cools
--as it does when the can is immersed in cold water--it turns into water. Water occupies less space than steam.
Assignment #3: Please write out your
explanation for the can's behavior.
So far you have observed something unexpected and formulated a hypothesis (plural
hypotheses) or guess as to what the explanation for what you saw might be. Your guess
might be right, or it might be wrong. Physicists rely on intuition to come up with
guesses, but the final arbiter
--always--is mother nature. They go to nature to see whether their hunches are
right or wrong. They do so because they know that they have been wrong in the past, that
every scientist that ever lived held some outlandish or wrong hypotheses, that human
beings are fallible, and that they themselves are no exception.
Non-physicists are often as curious as physicists. What is a rainbow, they may ask, and
come up with the reasonable explanation that it is God's symbolic message to humanity that
He will not flood the earth again. What is lightning, they may ask, and come up with the
reasonable answer that it is Zeus' secret weapon. Non-physicists often stop here, if the
explanation strikes them as reasonable, pretty, or if it is clothed by the trappings of
authority. The physicist, in contrast, proceeds to subject his hypotheses to empirical
(observational) tests.
We invite you to do the same. Please carry out the following additional tests, and
after each test ask yourself: Can the hypothesis I formulated earlier (in #3 above)
explain the observed results, or do I need to abandon it in favor of a more reasonable
hypothesis? If so, what might this new hypothesis be?
Repeat exactly the same experiment as in Step I above with a second aluminum can, but
with one difference
--don't invert the can. Instead,
immerse it half way or so in the bowl of cold water right side up.
Assignment #4: What were your
observations? Do they support or disprove your initial hypothesis? If they are
inconsistent with your hypothesis, can you come up with another?
| Fill up a large bowl with water. Immerse a small
drinking glass in it so that the glass is completely filled up with water. While keeping
the glass under water, invert it so that its open side faces the bottom of the bowl.
Gradually raise the glass above the waterline, still keeping its mouth beneath the
surface. |
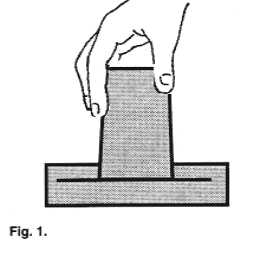 |
Assignment #5: Does the water run out
or stay in the glass? Why doesn't the water go down? What happens when the glass bottom is
lifted above the waterline? (Be sure to keep the glass vertical). Does this have anything
to do with the explanation for the collapsing can?
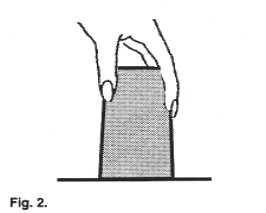
| Place a card over the open top of a glass filled
to the brim with water so that the card covers it completely. Slowly invert the glass
(upside down) while holding the card, and then let go of the card. |
|
Assignment #6: Describe what happened.
| Remove the tab from an empty aluminum soda can.
With the aid of a nail or some sharp object, make a small hole on the can's side near its
bottom. Close the hole with one finger and fill the can with water. |
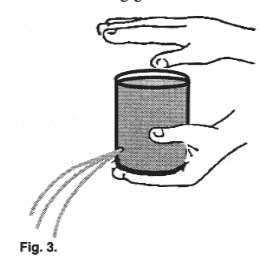 |
Assignment #7: Lift the finger from the hole?
What happens?
Assignment #8: Now, while the water is
running from the bottom hole, cover the top hole firmly and completely with your
thumb. What happens to the flow of water?
Assignment #9: While holding the side
hole, fill up the can again. Turn it upside down. Does the water come out from the main
opening? Does it flow as if from a tap or a hose, or does it gurgle? Repeat the above, but
now let go of the side hole while the can is upside down. Does the water gurgle or freely
flow?
Assignment #10: You now have a more
impressive body of observations to go upon than you had at the start. Are they all
consistent with your initial hypothesis? If not, can you think of one neat way to
explain all these observations? Can you think of additional ways of testing your
hypothesis?
Note: Assignment #10 is the KEY QUESTION of the entire exercise. Please try to come up
with an explanation that would tie all your observations into one coherent whole. If you
succeed, and if you didn't know the answer beforehand, you have every reason to be proud
of yourself
--it took science thousands of years to come
up with a coherent theory. If you arrive at a wrong theory, that's just as good--it's curiosity and the honest effort that count. The subject will be
taken up in great detail later on in this class.
Assignment 11: Your study of the
temperamental soda can is typical of science as a whole. What does your experiment tell
you about the nature of science? What does it tell you about the role in scientific
inquiries of observations, of guesses as to what the causes of these observations might
be, of actual tests of these guesses, and of creative imagination?
End of Exercise
A Guided Discovery Exercise for Introductory Physics Labs: A Note
To Science Teachers
The time-honored practice of incorporating a laboratory experience in introductory
physics classes has often come under fire (for a recent review, see Ref. 1). On the one
hand, there are those who feel that any effort to teach experimental physics without
engaging the learner in hands-on experiences is equivalent to teaching swimming
exclusively on dry land. On the other hand, there are those who feel that any similarity
between actual experimental work and the traditional physics laboratory is purely
coincidental, and that, given the costs of a physics laboratory experience and its
questionable effectiveness, the whole thing should be given up as a bad job.
In-between these two camps, there are those who feel that hands-on experiences ought to
form an integral part of physics instruction, but that this experience must take into
account (i) insights from cognitive and educational psychology, and (ii) the lukewarm
feedback from countless veterans of the traditional physics laboratory. That is, instead
of throwing out the baby with the bath water, these people suggest that we need to retain
--albeit with some meaningful modifications--the introductory physics lab.
We have presented elsewhere a few departures from traditional practices.
2-3 Here we
should like to describe another non-conventional--and
highly effective--approach which we have successfully
employed in our introductory physical science classes over the past four years.
By choosing to exemplify the self-discovery approach with a well-known experiment, we
hope to (i) illustrate our approach in terms that are already familiar to the reader and,
to (ii) show that many traditional laboratory demonstrations can be readily re-designed.
Because the exercise manual is reproduced in the enclosed appendix, we only need to
highlight some of its basic features.
We use a variation of the collapsing can exercise.
4-5 This take-home exercise is based
on self-explanatory instructions. The written guidelines begin with a thought experiment:
What will happen if you place a few teaspoons of water in an empty soda can, bring the
water to a boil, then partially immerse the can upside down in a bowl of cold water? After
students put down their expectation on paper, they are asked to carry out this procedure.
Most are surprised, delighted, and perplexed by the collapse of the can.
The key feature of this exercise, then, is self-discovery. Students are not told what
to expect. Instead, they are presented with a problem (a collapsing can), asked to
formulate an initial hypothesis in an effort to explain this problem, told to collect and
generate additional data which may throw light on their hypothesis, encouraged to revise
their hypothesis and, if necessary, formulate a new hypothesis which successfully pulls
the various observational threads together. They are then asked to generate, if they can,
a test of their final hypothesis. Students are then asked to draw some generalizations
about the nature of scientific discovery from their own similar experiences with the
collapsing can. The instructions make it clear that they are not expected to come up with
correct answers. The important thing, they are told, is that they grapple with the
material and strive to explain it as best they can on their own, without the assistance of
texts or friends. In other words, in this exercise, students abandon their traditional
role as passive learners and are guided along an active process of discovery.
After their lab report has been written, students are introduced to the process of
scientific discovery in lectures and readings
6 and to the historical development of the concept of atmospheric
pressure.7 Needless to say, their own hands-on earlier experiences help them
assimilate these theoretical concepts.
This exercise is conducted at home and costs next to nothing. It often becomes a topic
of conversation, generating considerable interest among family and friends. This vivid,
self-reliant experience is more likely to overcome naive misconceptions regarding the
nature of suction. It succeeds in demonstrating the commonsense nature of science, its
affinity to puzzle-solving, and its fun-filled nature. Although we are unable to document
this claim, this simple exercise seems to alleviate some of our students' negative
preconceptions and anxieties about physics. It gives students confidence in their own
abilities and shows them that they can satisfactorily comprehend some important physical
concepts. This exercise, and the class discussion of atmospheric pressure and the
scientific method which follow it, serve as a springboard to a discussion of the
applications of science: in this case, the mercury barometer and altimeter, their
principles of operation, and their social utility. Above all, this exercise provides a
hands-on, engaging illustration of the nature of scientific inquiry.
Needles to say, and depending on one's inclinations and background, a similar approach
can be applied to many other topics. Such a self-discovery approach, when used alongside
other traditional and non-traditional approaches, can enrich the quality of introductory
physics labs.
References
1. A. B. Arons, "Guiding insight and inquiry
in the introductory physics laboratory,"
Phys.
Teach. 31, 278-282 (1993).
2. M. Nissani, "A hands-on instructional approach
to the conceptual shift aspect of scientific discovery."
J. Coll. Sci. Teach. 19, 105-107 (1989).
3. M. Nissani, "A class exercise for teaching the
genetic code."
Sci. Teach., 56
(No. 3), 76-78 (1989).
4. P. G. Hewitt,
Conceptual Physics, sixth edition, (Scott, Foresman, and Company,
Glenview, 1989).
5. J. E. Stewart, "The collapsing can
revisited,"
Phys. Tea. 29,
144 (1991).
6. One delightful and readable account can be found
in: I. M. Copi,
Introduction to Logic,
seventh edition (Macmillan, London, 1986), pp. 492-505.
7. Here we use the classical account of: J. B. Conant,
Science and Common Sense, (Yale, New
Haven, 1951), pp. 63-96.